Limiting excess reactants reactant mass example reaction stoichiometry chemistry moles chapter much produced remains after given chemical products hcl two Difference between limiting reagent and excess reagent Excess reactant
Limiting and Excess Reactants, Basic Process and Calculations - YouTube
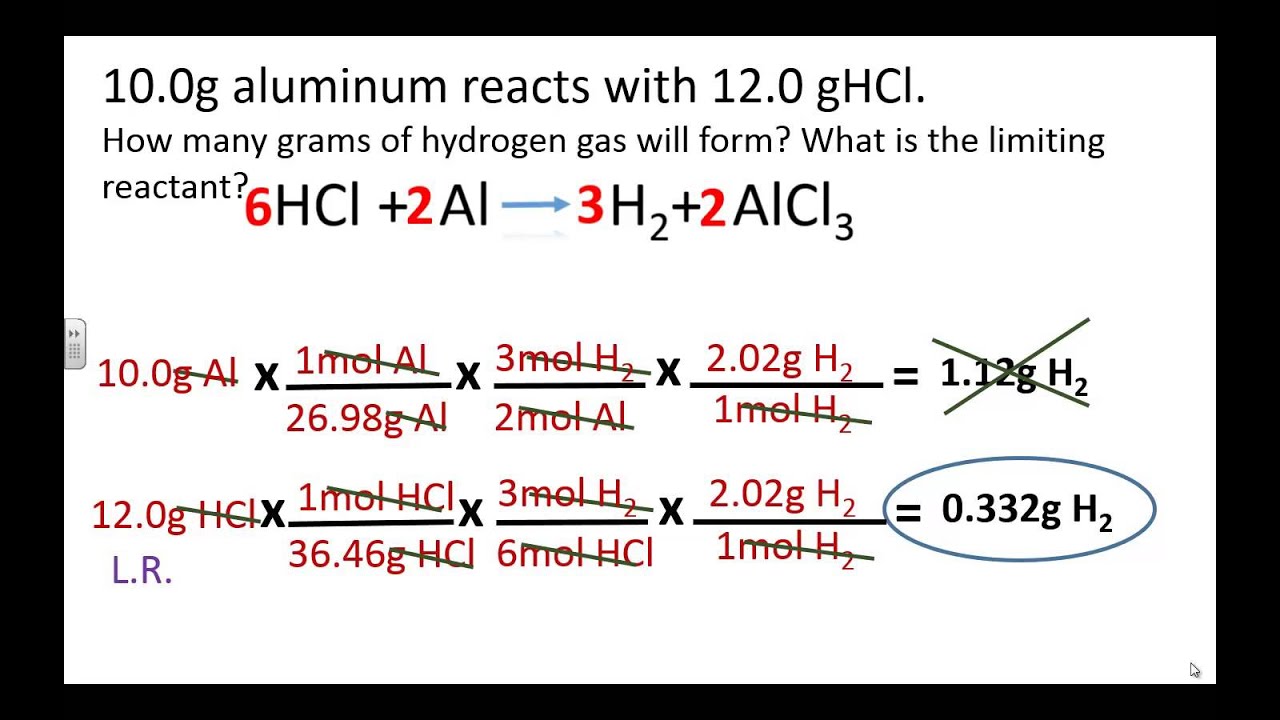
Limiting and excess reactants, basic process and calculations
Limiting reactant
How to find a limiting reactant| reactant in excess #igcse #gcse #aqa #Excess limiting reactants Limiting reactant example yield theoretical which ppt powerpoint presentation nh3 gas mol when analogy slideserveExcess reactant find left chemistry amount over.
19+ excess reagent calculatorFinding limiting and excess reagents How to calculate limiting reagent and excess reagentHow to determine limiting reactant.

How to calculate excess reactant
Limiting reagents reactant reactants example calculations reaction based mass need equation produced o2 co2 ppt powerpoint presentation formed h2o maximumLimiting reactant and limiting reagent Limiting reagent reactant definition examples and proLimited reagent worksheet answers.
How to determine limiting reactantLimiting reagent How to find the limiting reactant (my shortcut)How to determine limiting reactant.

How to calculate limiting reagent and excess reagent
Limiting reactantDetermining amount of excess reactant leftover Limiting reactant practice problemsHow to calculate limiting reagent and excess reagent.
Excess limiting reactantsLimiting reactant and percent yield worksheet answers Hc: unit 7 limiting and excess reactantsWhat is the limiting reactant? explained with examples.

Excess limiting reagents finding
Difference between limiting reactant and excess reactantHow to calculate limiting reagent and excess reagent Limiting & excess reactant example #1How to find the amount of excess reactant that is left over.
How to determine limiting reactantsExcess reactant limiting example Question video: recalling the definition of limiting reagentLimiting reactant problems practice.

Limiting reactant
.
.